Making Prevention Possible
Innovative proprietary preemptive screening technologies from CDx help improve patient outcomes and reduce healthcare costs for payers.
Innovative technology that reliably detects dysplasia and makes cancer prevention possible.
Learn MoreStay informed about your health and the benefits of advanced technology that finds precancerous cells.
Learn MoreInnovative proprietary preemptive screening technologies from CDx help improve patient outcomes and reduce healthcare costs for payers.
*Ruberstein JH Risk Factors for Barrett’s esophagus. Curr Opin Gatrone
While chronic heartburn (or GERD) is the most common risk factor for developing Barrett’s esophagus, other at-risk groups include:
Males
Obese patients
Tobacco users
Those >50 years of age
The cost to payers for screening and surveillance of Barrett's esophagus continues to increase yearly. Yet, the 5-year survival of esophageal adenocarcinoma remains miserable, in part, due to the limitations of the Seattle protocol.
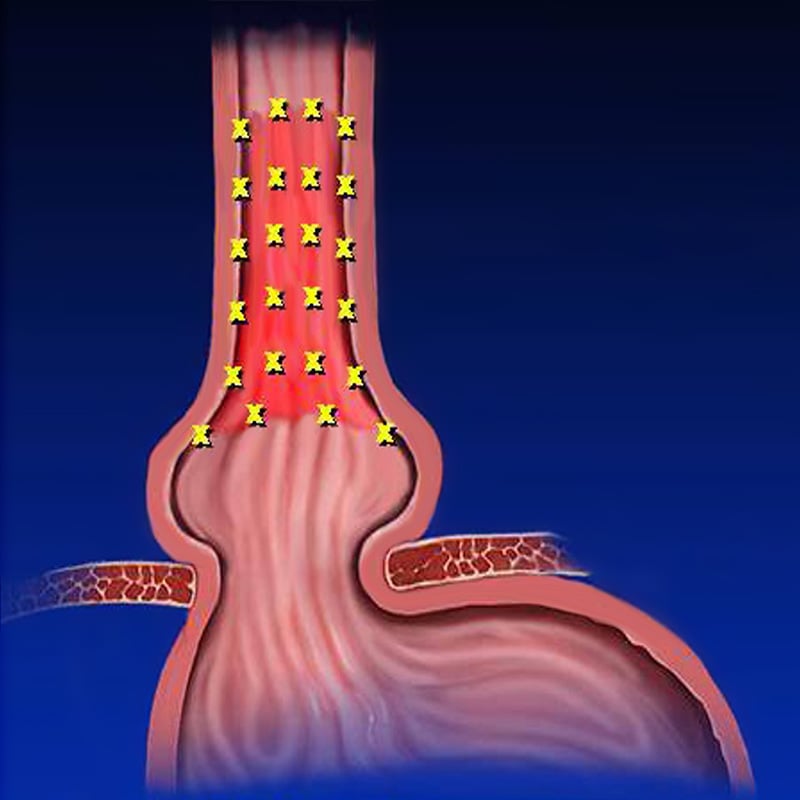
The Seattle random forceps biopsy protocol does not reliably detect Barrett’s esophagus or dysplasia, which are precursors to esophageal adenocarcinoma.
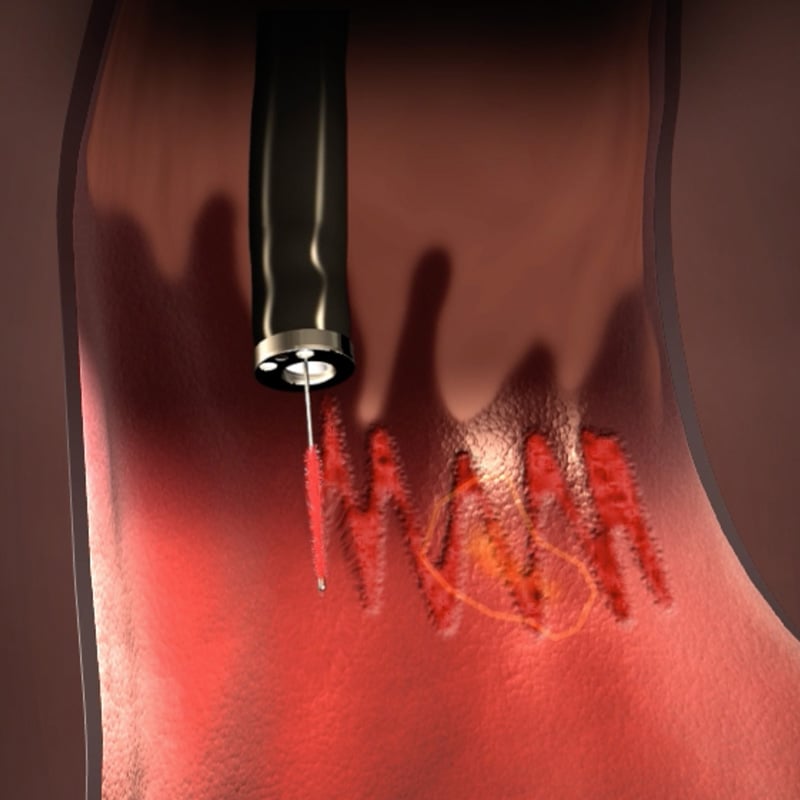
WATS3D (Wide-Area Transepithelial Tissue Sampling with computer-assisted 3D analysis), when used as an adjunct to standard biopsy sampling, has been shown to reliably detect unhealthy cells before they develop into cancer.
WATS3D is endorsed for both screening and surveillance of patients with suspected or known Barrett's esophagus , as an adjunct to routine biopsies, in the recently published Guidelines of the American Society for Gastrointestinal Endoscopy (ASGE).

“In patients with known or suspected BE, we suggest using WATS3D in addition to WLE with Seattle protocol biopsy sampling compared with WLE with Seattle protocol biopsy sampling alone”
*ASGE guideline on Barrett’s (GI Endosc 90(3) 2019 page 351)
EAC is preventable if detected and treated early. WATS3D has demonstrated the ability to improve patient outcomes by having a direct impact on the clinical management in 97% of patients diagnosed with BE, LGD or HGD.
Clinical utility of wide-area transepithelial sampling with three-dimensional computer-assisted analysis (WATS3D) in identifying Barrett’s esophagus and associated neoplasia.
Vivek Kaul, Seth Gross, F Scott Corbett, Zubair Malik, Michael S Smith, Christina Tofani, Anthony Infantolino
Diseases of the Esophagus, July, 01 2020
https://doi.org/10.1093/dote/doaa069
More than half of the US population is now over the age of 50, an age that is at increased risk for developing esophageal adenocarcinoma. Detecting dysplasia early and treating, it has the potential to reduce the incidence of this deadly disease. The addition of WATS3D per 1,000 patients results in approximately 3 fewer cancers and 3 fewer cancer-related deaths.
Wide Area Transepithelial Sampling with Computer-Assisted Analysis (WATS3D) Is Cost-Effective in Barrett’s Esophagus. Screening. Singer ME. Gross S, Kaul V, Smith MS. 449. Digestive Diseases and Sciences: June 23, 2020.
https://doi.org/10.1007/s10620-020-06412-1
WATS3D when used adjunctively to standard forceps biopsy is more cost-effective for Barrett's esophagus screening compared to forceps biopsy alone. Screening with WATS3D costs an additional $1,219 and produced an additional 0.017 QALYs, for an ICER of $71,395/QALY.
Wide Area Transepithelial Sampling with Computer-Assisted Analysis (WATS3D) Is Cost-Effective in Barrett’s Esophagus. Screening. Singer ME. Gross S, Kaul V, Smith MS. Digestive Diseases and Sciences: June 23, 2020.
https://doi.org/10.1007/s10620-020-06412-1
| CPT Code | CPT Descriptor | Maximum Daily Units |
|---|---|---|
| 88104 | Cytopathology, fluids, washings or brushing, except cervical or vaginal | 1 |
| 88305 | Surgical pathology, gross and microscopic examination | 1 |
| 88312 | Special stain including interpretation and report | 1 |
| 88361 | Morphimetric analysis, tumor immunohistochemistry (e.g., Her2nue, estrogen receptor/progesterone receptor), quantitative or semi-quantitative, each antibody, using computer-assisted technology | 4 |
|
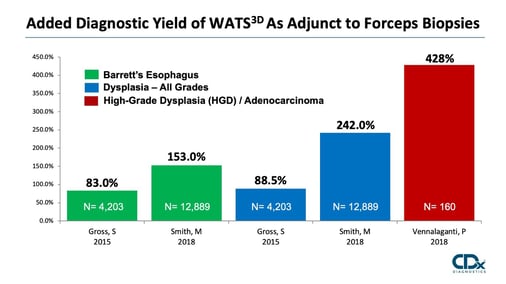
Study/Year: |
# of Patients: |
Study Design: |
Study Population: |
Main Results: |
|
Smith et al. |
12,899 |
Prospective Randomized |
Community Based |
⇪Barrett’s 153% ⇪Dysplasia 242% |
|
Gross et al. |
4,203 |
Prospective |
Community Based |
⇪Barrett’s 83% ⇪Dysplasia 89% |
|
Vennalaganti et al. |
160 16 Med Centers |
Prospective Randomized |
Academic Centers (High Risk) |
⇪High-Grade Dysplasia 428% |
|
Anandasabapathy et al. |
151 |
Prospective |
Academic Centers |
⇪Dysplasia 42% |
|
Johanson et al. |
1,266 |
Prospective Randomized |
Community Based |
⇪Barrett’s 70.5% ⇪Dysplasia 87.5% |
For more information about our products and services or general questions, we are here to help.
©2026 CDx Diagnostics. All Rights Reserved.